「领域快报」是AiBrain筹备的特别栏目,是由海内外知名高校的一线青年科研工作者(博士后、PI)精选的领域科研动态,旨在为学科融合、交叉合作提供平台和机遇。
作者:脑怼怼 复旦大学博士后
「领域快报」神经炎症及免疫
神经代谢、内分泌及内稳态
Brain Behavior and Immunity
PEA干预高脂饮食导致焦虑的神经免疫机制
Brain Behavior and Immunity (IF 7.217)
高脂饮食(high-fat-diet, HFD)除了引起肥胖等代谢表型外,还会导致肠道屏障完整性破坏。在此条件下通过肠道屏障进入循环的大分子会引发长期低水平的炎症。这类低水平炎症增加循环中游离脂肪酸和细胞因子的含量,对外周和中枢都造成影响,从而导致神经性炎症。长期的HFD不仅使下丘脑处于炎症状态,还会影响皮层、杏仁核和海马等情绪相关脑区的免疫状态,这可能是肥胖和焦虑之间存在共病的神经机制。
既往研究发现棕榈先乙醇酰胺(Palmitoylethanolamide, PEA)作为内源性的脂质介质,可以被过氧化物酶体增殖物激活受体(peroxisome proliferator-activated receptor, PPAR-α)激活,并且可以通过调节肥胖小鼠模型中下丘脑瘦素信号和摄食行为的神经环路来改善小鼠肥胖等代谢表型。
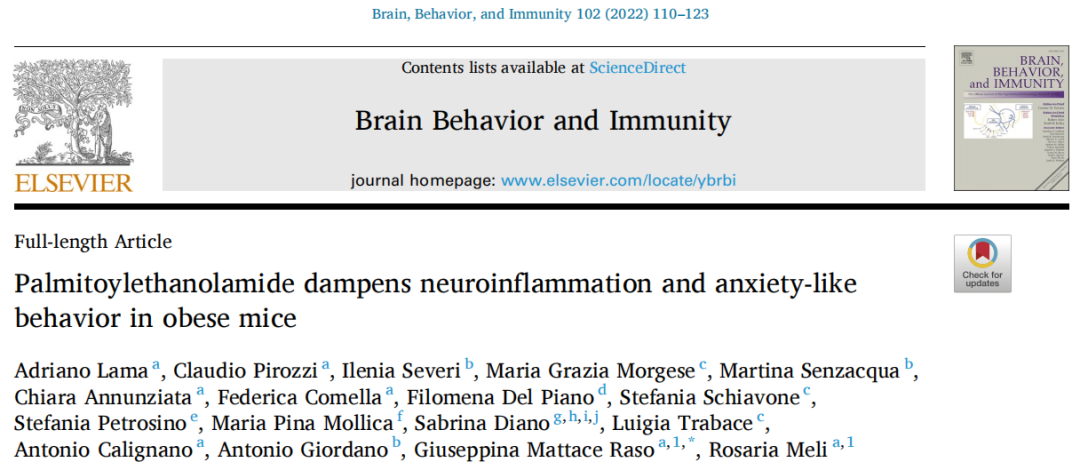
本研究于2022年3月发表在Brain Behavior and Immunity杂志,主要关注PEA在焦虑行为中的作用及机制。作者发现HFD喂养的小鼠表现出焦虑样行为,并且伴随杏仁核多巴胺周转率和GABA水平的降低。
PEA处理能够改善小鼠焦虑,使杏仁核多巴胺周转和GABA水平恢复正常。此外PEA还能抵抗HFD诱发的炎症和下丘脑损伤,抑制HFD小鼠海马星形胶质细胞和小胶质细胞的增殖,减轻神经炎症引起的血脑屏障完整性受损。
进一步以体外培养的SH-SY5Y细胞作为代谢性炎症的细胞模型,探究PEA对神经炎性反应的作用机制,研究发现PEA具有抗神经炎症反应和纠正线粒体功能异常的作用,且该作用由PPAR-α所介导。
★

本文通讯作者是意大利那不勒斯费德里克二世大学医学院的G. Mattace Raso教授,她的研究领域涉及内分泌药理学和免疫药理学,并关注外周和大脑双向沟通对代谢和免疫的调控。
★
评语:
肥胖相关的代谢疾病和焦虑抑郁等情绪疾病的共病率非常高,治疗抑郁和焦虑的药物也通常会引起肥胖的代谢表型,因此解析代谢和焦虑的共病机制并找到有效的治疗方法至关重要。本研究聚焦于HFD引发的肥胖和焦虑,作者拓展了PEA除治疗代谢表型之外,对焦虑的改善作用。作者通过大脑多个脑区的炎症状态和血脑屏障的完整性揭示了PEA干预肥胖性焦虑的神经免疫机制,并通过体外细胞培养探究其分子机制。
关键词:
high-fat diet; Palmitoylethanolamide; hypothalamic; amygdala; hippocampus; anxiety
文章链接DOI:
https://linkinghub.elsevier.com/retrieve/pii/S0889-1591(22)00037-X
Abstract
High-fat diet (HFD) consumption leads to obesity and a chronic state of low-grade inflammation, named metainflammation. Notably, metainflammation contributes to neuroinflammation due to the increased levels of circulating free fatty acids and cytokines. It indicates a strict interplay between peripheral and central counterparts in the pathogenic mechanisms of obesity-related mood disorders. In this context, the impairment of internal hypothalamic circuitry runs in tandem with the alteration of other brain areas associated with emotional processing (i.e., hippocampus and amygdala). Palmitoylethanolamide (PEA), an endogenous lipid mediator belonging to the N-acylethanolamines family, has been extensively studied for its pleiotropic effects both at central and peripheral level. Our study aimed to elucidate PEA capability in limiting obesity-induced anxiety-like behavior and neuroinflammation-related features in an experimental model of HFD-fed obese mice. PEA treatment promoted an improvement in anxiety-like behavior of obese mice and the systemic inflammation, reducing serum pro-inflammatory mediators (i.e., TNF-α, IL-1β, MCP-1, LPS). In the amygdala, PEA increased dopamine turnover, as well as GABA levels. PEA also counteracted the overactivation of HPA axis, reducing the expression of hypothalamic corticotropin-releasing hormone and its type 1 receptor. Moreover, PEA attenuated the immunoreactivity of Iba-1 and GFAP and reduced pro-inflammatory pathways and cytokine production in both the hypothalamus and hippocampus. This finding, together with the reduced transcription of mast cell markers (chymase 1 and tryptase β2) in the hippocampus, indicated the weakening of immune cell activation underlying the neuroprotective effect of PEA. Obesity-driven neuroinflammation was also associated with the disruption of blood-brain barrier (BBB) in the hippocampus. PEA limited the albumin extravasation and restored tight junction transcription modified by HFD. To gain mechanistic insight, we designed an in vitro model of metabolic injury using human neuroblastoma SH-SY5Y cells insulted by a mix of glucosamine and glucose. Here, PEA directly counteracted inflammation and mitochondrial dysfunction in a PPAR-α-dependent manner since the pharmacological blockade of the receptor reverted its effects. Our results strengthen the therapeutic potential of PEA in obesity-related neuropsychiatric comorbidities, controlling neuroinflammation, BBB disruption, and neurotransmitter imbalance involved in behavioral dysfunctions.
Cell Metabolism
下丘脑POMC中孕烯醇酮合成异常介导代谢疾病状态下的认知损伤

Cell Metabolism (IF 27.287)
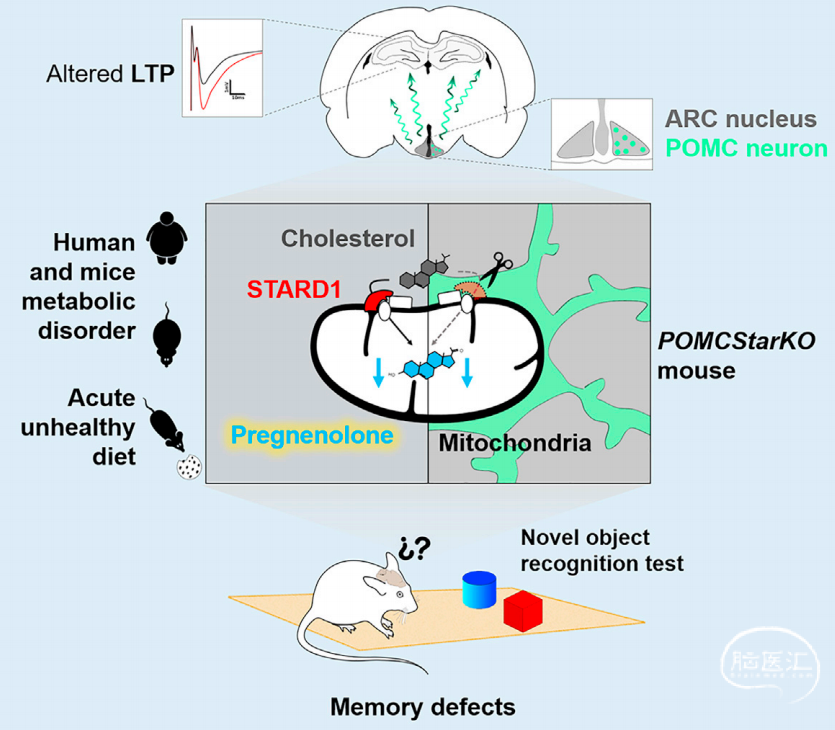
肥胖和2型糖尿病会使老年痴呆症的患病率成倍增加,大脑中胰岛素抵抗被认为是链接代谢疾病和认知退化的重要因素。近年来的研究发现大脑胆固醇代谢紊乱和胰岛素抵抗存在复杂的相互作用,并且糖尿病和衰老小鼠模型的大脑表现出与认知表型关联的胆固醇合成减少。然而神经类固醇对认知功能改变的贡献机制还需要进一步阐释。
本研究中于2022年2月发表在Cell Metabolism杂志上。作者对C57BL/6小鼠进行为期4天的高脂高糖喂养(high-fat high sucrose western diet, WD),这种短期处理并不会带来显著的代谢失衡,但通过巴恩斯迷宫和新颖物体识别实验发现其空间记忆虽然没有受影响,但物体识别能力已经产生了异常。同时检测了学习记忆关键脑区海马的电生理活动,发现高脂高糖的食物使海马LTP大幅降低。此外作者聚焦到胆固醇代谢物孕烯醇酮(线粒体类固醇合成途径中的第一个中间体,也是几乎所有类固醇激素的前体),发现在海马和皮层孕烯醇酮的含量不受高脂高糖的影响,但弓状核中孕烯醇酮在高脂高糖处理后显著降低。进一步通过侧脑室给药发现急性注射孕烯醇酮就可以挽救小鼠物体识别的缺陷。
随后,作者通过敲除策略表明弓状核中POMC神经元的孕烯醇酮会影响小鼠的认知行为,并且是由自分泌途径而非胰岛素途径所介导。作者延长了高脂高糖的处理的时间使小鼠肥胖且代谢异常,得出与高脂高糖的喂养4天相似的结论,同时在人群的研究中也得到了验证。
★

本文通讯作者为西班牙August Pi i Sunyer 生物医学研究所,代谢神经调控课题组的Marc Claret教授和Sara Ramı´rez博士。作者致力于研究调节摄食、体重和代谢特定神经环路的分子机制,特别是下丘脑相关的神经机制,同时也关注代谢和情绪及认知之间关联的机理。
★
评语:
本文通过控制WD喂养的时间,构建了代谢没有明显异常但认知层面已经出现问题的小鼠模型,并解析了孕烯醇酮在其中的神经机制,同时也在长期WD喂养出现代谢异常的小鼠中进行了验证。对代谢状态的精确调控分离出代谢状态的变量,提示高脂高糖饮食在造成肥胖之前就已经通过影响神经活动来损伤认知功能。表明除了通过达到肥胖结局后影响免疫和胰岛素敏感性从而影响智力的机制之外,还存在其他的机制。另一方面这篇文章发现POMC神经元除了调控摄食、能量稳态之外的功能,拓展了我们对经典“抑食神经元”的认识。
关键词:
Pregnenolone; Arc; POMC; recognition
文章链接 :
https://doi.org/10.1016/j.cmet.2021.12.023
Abstract
Obesity and type 2 diabetes are associated with cognitive dysfunction. Because the hypothalamus is implicated in energy balance control and memory disorders, we hypothesized that specific neurons in this brain region are at the interface of metabolism and cognition. Acute obesogenic diet administration in mice impaired recognition memory due to defective production of the neurosteroid precursor pregnenolone in the hypothalamus. Genetic interference with pregnenolone synthesis by Star deletion in hypothalamic POMC, but not AgRP neurons, deteriorated recognition memory independently of metabolic disturbances. Our data suggest that pregnenolone’s effects on cognitive function were mediated via an autocrine mechanism on POMC neurons, influencing hippocampal long-term potentiation. The relevance of central pregnenolone on cognition was also confirmed in metabolically unhealthy patients with obesity. Our data reveal an unsuspected role for POMC neuron-derived neurosteroids in cognition. These results provide the basis for a framework to investigate new facets of POMC neuron biology with implications for cognitive disorders.
在招岗位
2022 RECRUIT /
招募编译团队及审稿团队
岗位职责:撰写、审阅神经科学各领域的文献导读、领域速报。
岗位要求:神经科学领域四年级以上的博士生、博士后以及青年科研工作者。
AiBrain作者团队由海内外知名高校博士生、博士后,及已成立实验室的PI们组成;加入AiBrain,可以体验专业的投审稿系统、高效温馨的团队合作、丰富的文章栏目,以及有竞争力的丰厚报酬;能力突出者可以作为领域/栏目负责人,并配有岗位津贴。
欢迎有意者投递简历及个人作品!

微信号:AiBrainzhushou
邮箱:ai_brain@163.com


声明:脑医汇旗下神外资讯、神介资讯、脑医咨询、AiBrain所发表内容之知识产权为脑医汇及主办方、原作者等相关权利人所有。未经许可,禁止进行转载、摘编、复制、裁切、录制等。经许可授权使用,亦须注明来源。欢迎转发、分享




