「领域快报」是AiBrain筹备的特别栏目,是由海内外知名高校的一线青年科研工作者(博士后、PI)精选的领域科研动态,旨在为学科融合、交叉合作提供平台和机遇。
「领域快报」神经退行性疾病
ACS Nano
基于近红外光的纳米传感器:细胞内和脑内β-淀粉样蛋白的检测技术
ACS Nano (IF 15.881)
β淀粉样蛋白 (Aβ)的沉积发生在阿尔茨海默病 (AD)的早期阶段,但 Aβ 的早期检测一直以来都是难题。
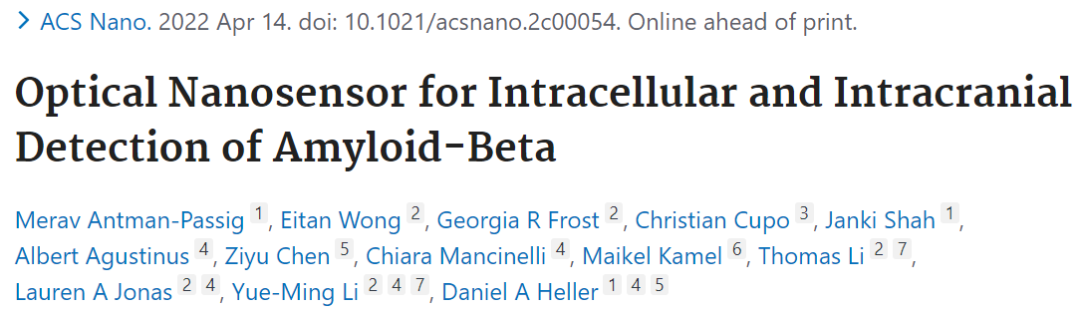
由于近红外光能够穿透颅骨,具有较强的组织穿透能力;本文便设计了一种基于近红外光的纳米传感器(Aβ-carbon nanotube,ABCNT),不仅能够在活细胞中检测到胞内 Aβ,还能在体实验中检测出脑内的Aβ。该传感器由Aβ 修饰的单壁碳纳米管构成,传感器表面修饰的Aβ 与神经组织内的Aβ相互作用即可引发探针响应,其作用原理在于修饰后的碳纳米管结合Aβ后,在近红外光照射后会产生溶致变色现象(Solvatochromic effect,溶剂极性变化引起染料分子的发射光谱产生不同的响应性,从而颜色改变)。因此,传感器能够追踪 Aβ在活细胞中积累,并在AD模型的老年小鼠颅内给药后表现出不同的响应。该技术能够应用于在体系统,探究 AD 发展中的 Aβ 神经毒性的分子机制。
评语:
本文开发的光学纳米传感器的核心特征是能够进行活细胞或者活体Aβ追踪,有效解决了正电子发射断层扫描/磁共振成像等方法无法探测早期Aβ的聚集,以及其它研究开发出的Aβ探针穿透深度不足的问题,这为Aβ相关的预临床研究提供了重要的研究方法。
关键词:
biosensor; carbon nanomaterials; fluorescence; nanocarbon; neurodegenerative disease
文章链接 DOI:
10.1021/acsnano.2c00054
PMID:
35420796
Abstract
Amyloid-beta (Aβ) deposition occurs in the early stages of Alzheimer's disease (AD), but the early detection of Aβ is a persistent challenge. Herein, we engineered a near-infrared optical nanosensor capable of detecting Aβ intracellularly in live cells and intracranially in vivo. The sensor is composed of single-walled carbon nanotubes functionalized with Aβ wherein Aβ-Aβ interactions drive the response. We found that the Aβ nanosensors selectively responded to Aβ via solvatochromic modulation of the near-infrared emission of the nanotube. The sensor tracked Aβ accumulation in live cells and, upon intracranial administration in a genetic model of AD, signaled distinct responses in aged mice. This technology enables the interrogation of molecular mechanisms underlying Aβ neurotoxicity in the development of AD in living systems.
Nature communications
小鼠小胶质细胞的NF-κB 驱动tau蛋白扩散和tau蛋白毒性

Nature communications (IF 14.919)
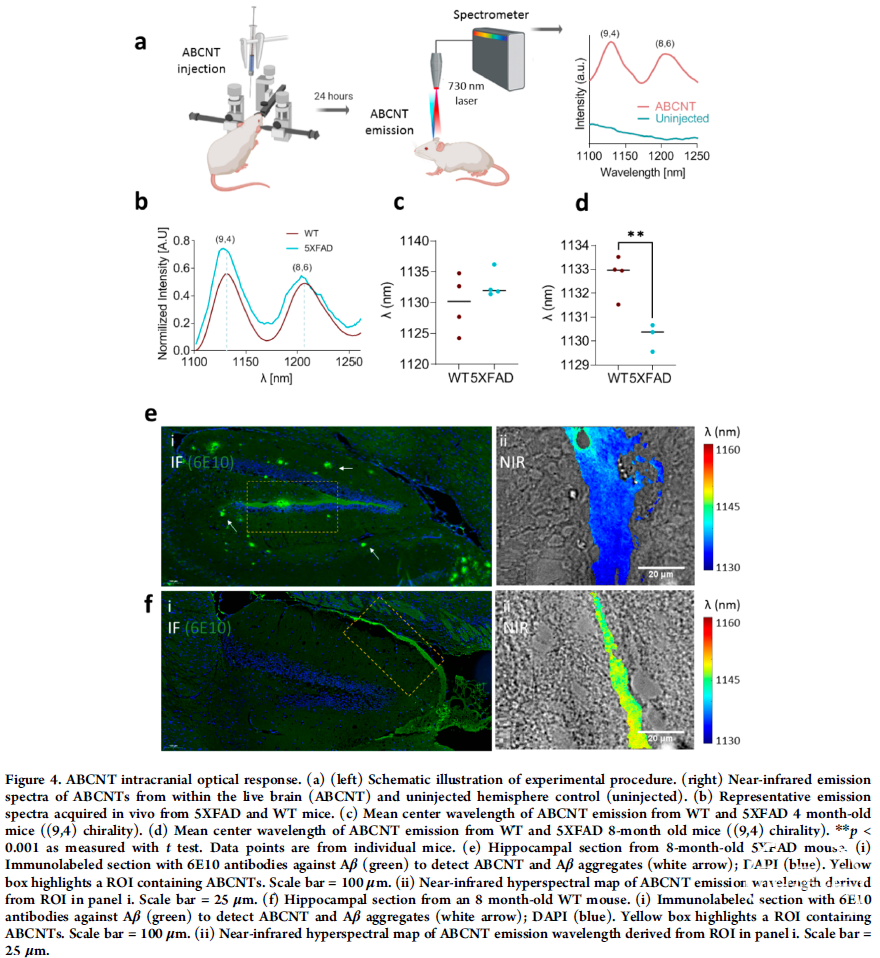
小胶质细胞的激活是tau蛋白病变(包括阿尔茨海默病)的显著病理特征之一。小胶质细胞的激活如何导致 tau蛋白毒性度仍不清楚。
本文展示了活化 B 细胞 (NF-κB) 的核因子 kappa-轻链增强子,由 tau 蛋白激活的信号传导,驱动小胶质细胞介导的 tau 蛋白扩散和tau蛋白毒性。如果增强小胶质细胞 NF-κB 的激活,减少其失活,tau便可在年轻的 PS19 小鼠中播种和传播。如果抑制 NF-κB ,便可将致病性 tau 纤维保留于初级小胶质细胞中,同时减少其从初级小胶质细胞释放,并挽救小胶质细胞自噬缺陷。
而对于衰老的PS19 小鼠,抑制小胶质细胞 NF-κB ,能够挽救 tau 介导的学习和记忆缺陷,恢复转录组的变化,同时增加了神经元 tau 包涵体。作者又通过单细胞 RNA-seq ,揭示NF-κB 失活会导致小胶质细胞的 tau 相关疾病状态减轻,组成型 NF-κB 激活则会导致小胶质细胞细胞激活。综上所述,本文发现了在tau蛋白病变中,小胶质细胞 NF-κB 信号传导通路介导了 tau 蛋白扩散和 tau蛋白毒性。
评语:
NF-κB信号通路的失调在AD病理研究中被广泛证实,来自加州大学旧金山分校的Li Gan课题组之前的研究发现SIRT1(一种组蛋白去乙酰酶)能够抑制NF-κB信号通路,从而避免小胶质细胞介导的Aβ神经毒性(Chen et al., 2005)。在本研究中,该课题组从新的角度探索了小胶质细胞NF-κB信号通路在tau蛋白病变中的作用极其机制,并发现对于tau病变小鼠,小胶质细胞中NF-κB的激活对于小胶质细胞介导的tau蛋白扩散和空间记忆缺陷非常重要,从而提出了一个针对tau蛋白病变的潜在治疗策略。
关键词:
microglia, tauopathy, NF-κB, tau propagation, tau toxicity
文章链接 :
https://doi.org/10.1038/s41467-022-29552-6
PMID:
35413950
Abstract
Activation of microglia is a prominent pathological feature in tauopathies, including Alzheimer's disease. How microglia activation contributes to tau toxicity remains largely unknown. Here we show that nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling, activated by tau, drives microglial-mediated tau propagation and toxicity. Constitutive activation of microglial NF-κB exacerbated, while inactivation diminished, tau seeding and spreading in young PS19 mice. Inhibition of NF-κB activation enhanced the retention while reduced the release of internalized pathogenic tau fibrils from primary microglia and rescued microglial autophagy deficits. Inhibition of microglial NF-κB in aged PS19 mice rescued tau-mediated learning and memory deficits, restored overall transcriptomic changes while increasing neuronal tau inclusions. Single cell RNA-seq revealed that tau-associated disease states in microglia were diminished by NF-κB inactivation and further transformed by constitutive NF-κB activation. Our study establishes a role for microglial NF-κB signaling in mediating tau spreading and toxicity in tauopathy.
Nature Reviews Neurology
对于早期AD患者临床试验招募工作,如何才能更加经济且高效?

Nature Reviews Neurology (IF 42.937)
当下,减缓阿尔茨海默病 (AD) 的进一步发展可能是我们这个时代最大且未被满足的医疗需求。尽管目前已有AD 疗法获得了 FDA加速批准(存在争议),我们仍迫切需要更有效和更容易获得的疗法。当前的共识是,为了在 AD 发展过程中进行有意义的治疗,治疗干预必须在疾病的早期阶段(临床前期或前驱期)开始。虽然已经开发出了针对这样的早期临床试验的方法,但识别和招募无症状或症状轻微的被试过程非常费时费力。
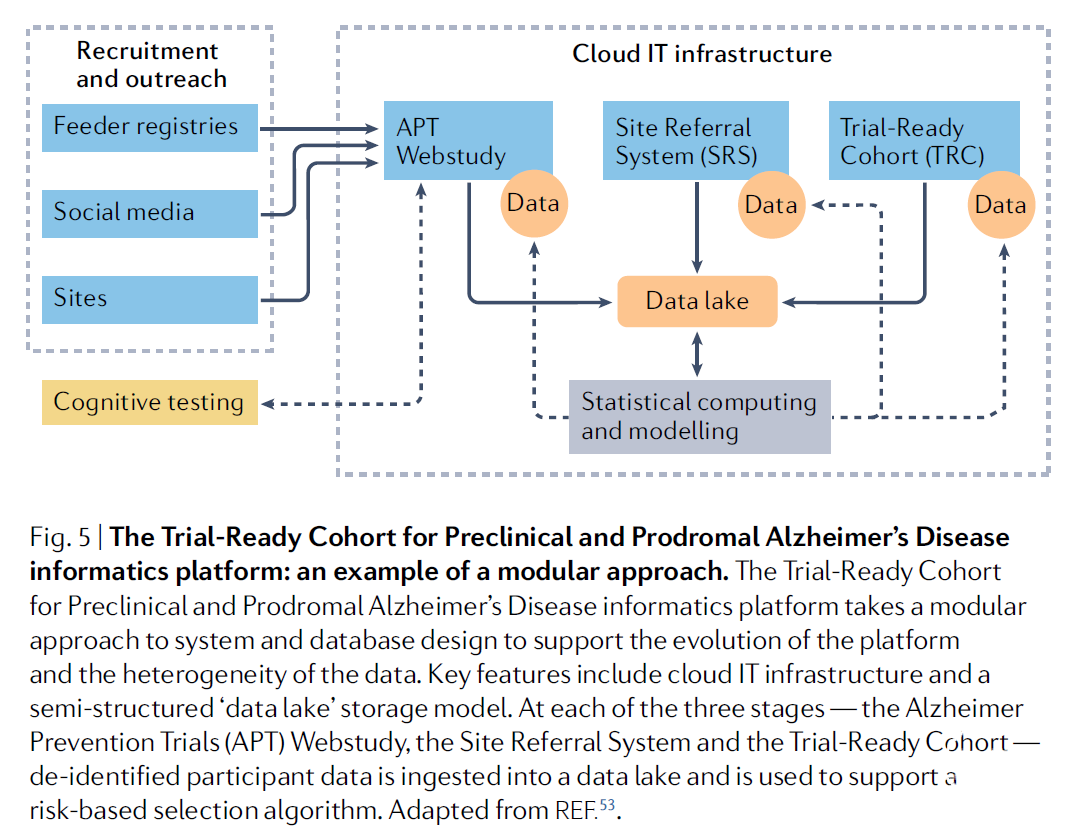
例如,在无症状AD患者的抗淀粉样蛋白治疗试验中(首例AD临床前期III 期临床试验),需要3.5 年时间和超过5,900 多种筛查来招募1,169 名参与者,并且为了提高招募效率并加速AD治疗进展,需要新的临床试验基础设施。北美、欧洲和亚洲正在合作通过建立临床前期和前驱期 AD 个体的试验就绪队列来解决这个问题。这些合作正在采用创新方法吸引目标人群,评估大脑淀粉样蛋白积累风险,选择参与者进行生物标志物研究并确定参与试验的资质。将来,这些计划可以提供有效的工具在早期阻止AD的发病进程。本文回顾了从 AD 试验就绪队列成立至今吸取的经验教训,旨在为当前和将来的试验招募工作提供更加高效、经济的信息。
评语:
试验就绪队列(Trial-ready cohorts)对于识别合格的早期AD临床试验参与者来说,是一种有效的策略。但是其具体实施过程仍有诸多困难,例如难以获得具有种族代表性的队列以及网上在线队列参与者容易流失。因此,需要更多的合作共同努力改善或者解决试验就绪队列策略的不足,助力更好的AD 疗法研发。
关键词:
AD, therapeutic intervention, clinical trials
文章链接 DOI:
10.1038/s41582-022-00645-6
PMID:
35379951
Abstract
Slowing the progression of Alzheimer disease (AD) might be the greatest unmet medical need of our time. Although one AD therapeutic has received a controversial accelerated approval from the FDA, more effective and accessible therapies are urgently needed. Consensus is growing that for meaningful disease modification in AD, therapeutic intervention must be initiated at very early (preclinical or prodromal) stages of the disease. Although the methods for such early-stage clinical trials have been developed, identification and recruitment of the required asymptomatic or minimally symptomatic study participants takes many years and requires substantial funds. As an example, in the Anti-Amyloid Treatment in Asymptomatic Alzheimer's Disease Trial (the first phase III trial to be performed in preclinical AD), 3.5 years and more than 5,900 screens were required to recruit and randomize 1,169 participants. A new clinical trials infrastructure is required to increase the efficiency of recruitment and accelerate therapeutic progress. Collaborations in North America, Europe and Asia are now addressing this need by establishing trial-ready cohorts of individuals with preclinical and prodromal AD. These collaborations are employing innovative methods to engage the target population, assess risk of brain amyloid accumulation, select participants for biomarker studies and determine eligibility for trials. In the future, these programmes could provide effective tools for pursuing the primary prevention of AD. Here, we review the lessons learned from the AD trial-ready cohorts that have been established to date, with the aim of informing ongoing and future efforts towards efficient, cost-effective trial recruitment.

声明:脑医汇旗下神外资讯、神介资讯、脑医咨询、AiBrain所发表内容之知识产权为脑医汇及主办方、原作者等相关权利人所有。未经许可,禁止进行转载、摘编、复制、裁切、录制等。经许可授权使用,亦须注明来源。欢迎转发、分享




