「领域快报」是AiBrain筹备的特别栏目,是由海内外知名高校的一线青年科研工作者(博士后、PI)精选的领域科研动态,旨在为学科融合、交叉合作提供平台和机遇。
「领域快报」神经代谢
Nature
肠道代谢物引起情绪障碍的神经机制

Nature (IF 49.962)
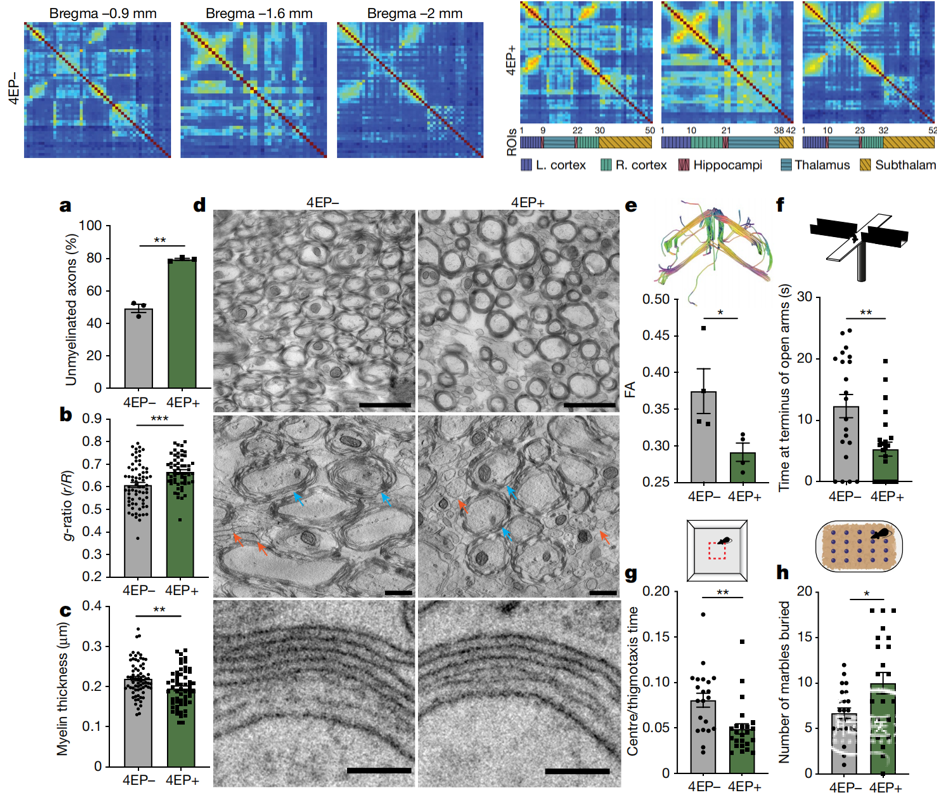
外界环境中感觉刺激和物质信息的整合对动物行为的塑造至关重要。胃肠道将摄入的食物代谢后产生的化合物可以传递到机体包括大脑在内的其他器官,因此胃肠道是接受环境中物质信息的关键部位。作者前期研究发现在自闭症患者和模型鼠的血液中微生物代谢物4-乙基硫酸酯(4EPS)异常升高,在本文中,作者深入解析了4EPS影响大脑并塑造行为的神经机制。首先作者鉴定了肠道微生物将食源酪氨酸转化成4EPS的生物合成途径,并运用生物工程改造微生物达到在无菌鼠中选择性产生4EPS的效果。研究发现肠道产生的4EPS可以通过血脑屏障进入大脑,并引起丘脑、海马、杏仁核、下丘脑、梨状皮质以及皮层的功能连接异常。对这些脑区进行测序分析发现少突胶质细胞功能相关的的基因表达受到影响。进一步研究发现4EPS使少突胶质细胞的成熟过程受损并阻碍髓鞘的形成,最终使小鼠表现出焦虑行为。药理学干预少突胶质细胞的成熟可以有效防止4EPS造成的行为缺陷。
评语:
我们知道肠道微生物通过分解产生的代谢物通过神经内分泌途径或肠-脑神经投射对大脑的发育和动物行为有重要的影响,但是其机制还没有得到明确的阐释。本文结合传统神经生物学技术和小动物影像技术对肠道微生物代谢产生的分子影响行为的神经机理展开研究,揭示了自闭症谱系障碍患者血液中异常的代谢物4EPS通过血脑屏障影响少突胶质细胞功能,损伤髓鞘形成,改变功能连接从而引起焦虑行为的机制,为神经发育障碍的情绪问题提供脑肠轴干预策略和机制研究。
关键词:
microbial metabolite 4-ethylphenyl sulfate (4EPS);oligodendrocyte;anxiety-like behaviours
文章链接 :
https://www.nature.com/articles/s41586-022-04396-8
Abstract
Integration of sensory and molecular inputs from the environment shapes animal behaviour. A major site of exposure to environmental molecules is the gastrointestinal tract, in which dietary components are chemically transformed by the microbiota and gut-derived metabolites are disseminated to all organs, including the brain. In mice, the gut microbiota impacts behaviour, modulates neurotransmitter production in the gut and brain, and influences brain development and myelination patterns. The mechanisms that mediate the gut–brain interactions remain poorly defined, although they broadly involve humoral or neuronal connections. We previously reported that the levels of the microbial metabolite 4-ethylphenyl sulfate (4EPS) were increased in a mouse model of atypical neurodevelopment. Here we identified biosynthetic genes from the gut microbiome that mediate the conversion of dietary tyrosine to 4-ethylphenol (4EP), and bioengineered gut bacteria to selectively produce 4EPS in mice. 4EPS entered the brain and was associated with changes in region-specific activity and functional connectivity. Gene expression signatures revealed altered oligodendrocyte function in the brain, and 4EPS impaired oligodendrocyte maturation in mice and decreased oligodendrocyte–neuron interactions in ex vivo brain cultures. Mice colonized with 4EP-producing bacteria exhibited reduced myelination of neuronal axons. Altered myelination dynamics in the brain have been associated with behavioural outcomes. Accordingly, we observed that mice exposed to 4EPS displayed anxiety-like behaviours, and pharmacological treatments that promote oligodendrocyte differentiation prevented the behavioural effects of 4EPS. These findings reveal that a gut-derived molecule influences complex behaviours in mice through effects on oligodendrocyte function and myelin patterning in the brain.
Nature neuroscience
肠道糖感受的迷走神经机制

Nature neuroscience (IF 24.884)
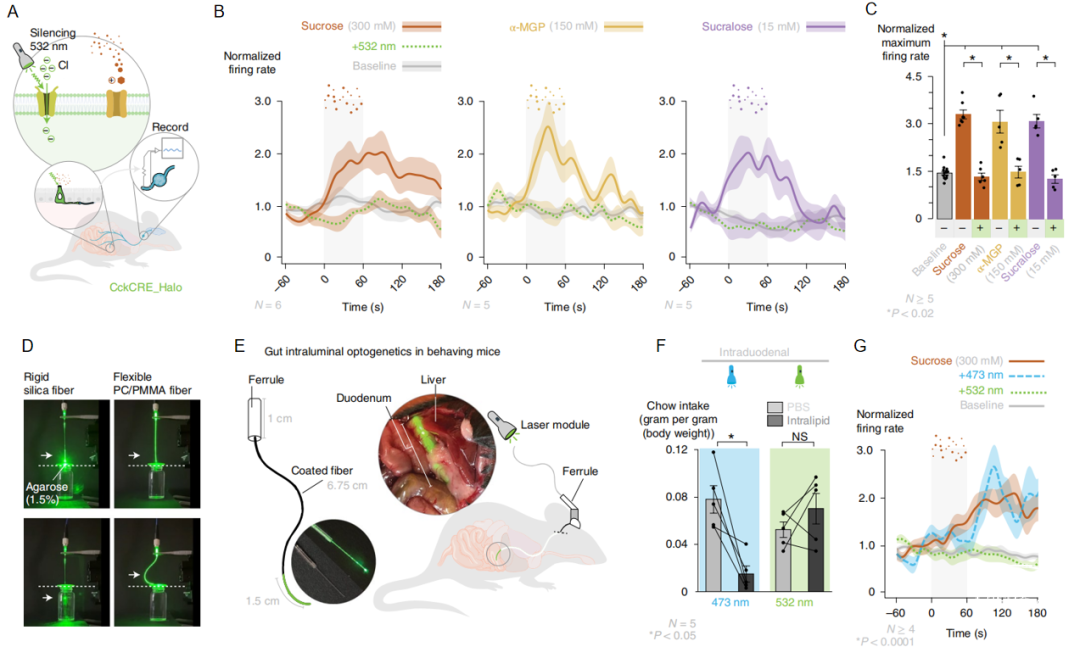
食物的能量和适口性都会影响动物对食物的偏好和选择,众多研究者都在实验动物中发现与人工甜味剂相比,动物会更偏好于可以被代谢产生能量的糖类,甚至在味盲小鼠中也能够产生相同的偏好。这说明机体摄入食物后存在细胞可以区出分糖类和人工甜味剂,但这一具体机制并不清楚。在本文中作者发现十二指肠处的迷走神经在接受到糖、人工甜味剂时神经活动明显增加,并且这一活动依赖于十二指肠中表达胆囊收缩素(cholecystokinin,CCK)的神经足细胞。进一步研究发现十二指肠的CCK神经足细胞通过甜味受体和葡糖糖钠转运体,将人工甜味剂和糖的信号传递到迷走神经,且这两类刺激的传递途径不同,人工甜味剂通过嘌呤能神经进行传递,而糖通过谷氨酸能神经系统进行传递。作者结合现代神经科学技术发展出独特的肠道光遗传学系统,解析了肠道快速识别糖类营养物质的神经机制,为糖摄入偏好的形成提供新机制研究。
评语:
领域内很早就发现动物会对可代谢性糖产生比人工甜味剂更强的偏好,甚至可以通过学习形成代谢驱动的糖偏好记忆。之前报道的机制解释为糖和人工甜味剂引起边缘系统不同亚区的多巴胺释放,此外也有研究揭示糖在摄入后胃肠道产生的代谢信号通过肠脑轴传到大脑从而形成对糖的偏好。本文发现了能够直接区别糖与人工甜味剂的迷走神经通路,提供了糖偏好形成的新机制。此外本文发展出肠道神经操控策略,弥补了传统光遗传硅光纤材料在肠道中易引起损伤的缺陷,打破了传统光遗传学在内脏器官使用的局限性。
关键词:
Sugar;artificial sweetness;vagus;preference
文章链接 :
https://www.nature.com/articles/s41593-021-00982-7
Abstract
Guided by gut sensory cues, humans and animals prefer nutritive sugars over non-caloric sweeteners, but how the gut steers such preferences remains unknown. In the intestine, neuropod cells synapse with vagal neurons to convey sugar stimuli to the brain within seconds. Here, we found that cholecystokinin (CCK)-labeled duodenal neuropod cells differentiate and transduce luminal stimuli from sweeteners and sugars to the vagus nerve using sweet taste receptors and sodium glucose transporters. The two stimulus types elicited distinct neural pathways: while sweetener stimulated purinergic neurotransmission, sugar stimulated glutamatergic neurotransmission. To probe the contribution of these cells to behavior, we developed optogenetics for the gut lumen by engineering a flexible fiberoptic. We showed that preference for sugar over sweetener in mice depends on neuropod cell glutamatergic signaling. By swiftly discerning the precise identity of nutrient stimuli, gut neuropod cells serve as the entry point to guide nutritive choices.
Molecular Psychiatry
外周内源性大麻素信号调控食物奖赏和能量平衡的肠脑沟通机制

Molecular Psychiatry (IF 15.992)
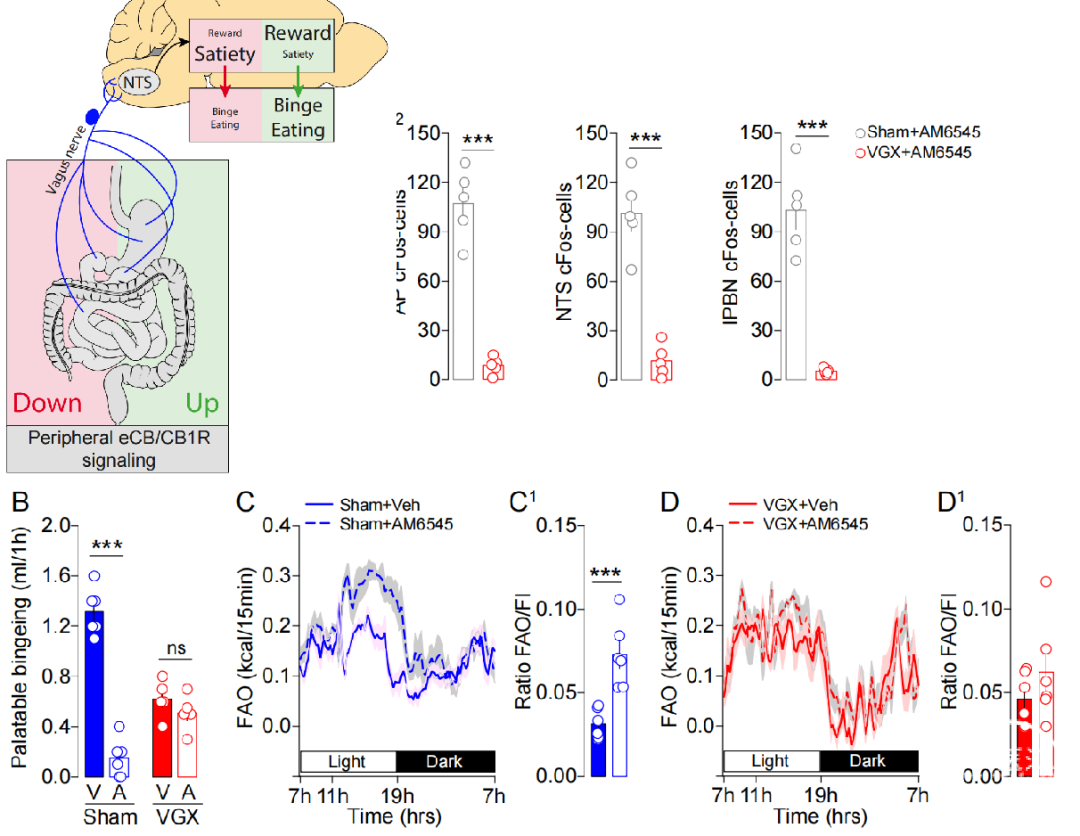
进食是非常复杂且保守的行为,受到能量平衡和奖赏信号整合后动态信息的调控。在可口、高热量食物普遍易得的今天,食物的奖赏效应成为塑造饮食习惯的重要因素。暴食症(binge eating)就是由食物奖励功能障碍引起的摄食不受控制的疾病,但是具体的生理和分子机制并不明确。本文作者构建了新型的由可口食物引发的暴食症小鼠模型,并确定了其摄食和代谢表型。作者进一步研究发现暴食模型小鼠产生的摄食适应性由外周的内源性大麻素信号(endocannabinoids, eCBs)所介导,并且依赖于迷走神经的完整。利用选择性的外周CB1受体拮抗剂会抑制奖赏诱导暴食症的发生,改变代谢效率,并且抑制边缘系统多巴胺环路的活动。本文揭示了eCBs信号通过控制迷走神经活动,上行控制代谢稳态和奖赏环路的神经反应从而调控奖赏驱动摄食行为的机制,提示eCBs可能是干预能量平衡和食物奖赏疾病的新靶点。
评语:
目前常用的暴食症造模方式是禁食结合提供高脂食物,这与临床上暴食症的产生方式相差较远,而本文作者所采用的行为范式是限时提供高度美味的、加糖加脂的牛奶混合物,来促进食物的奖赏作用,但不限制普通饲料的摄入。这种造模方式更加符合自然条件下人类暴食症的形成过程。此外本文还揭示了食物奖赏效应通过迷走神经将肠道信息传达大脑,引起大脑活动变化从而调控摄食行为的生理机制,丰富了我们对于肠脑轴沟通塑造饮食习惯的分子机制的认知。
关键词:
peripheral endocannabinoids (eCBs);gut-brain axis;vagus;binge eating
文章链接 :
https://www.nature.com/articles/s41380-021-01428-z
Abstract
The regulation of food intake, a sine qua non requirement for survival, thoroughly shapes feeding and energy balance by integrating both homeostatic and hedonic values of food. Unfortunately, the widespread access to palatable food has led to the development of feeding habits that are independent from metabolic needs. Among these, binge eating (BE) is characterized by uncontrolled voracious eating. While reward deficit seems to be a major contributor of BE, the physiological and molecular underpinnings of BE establishment remain elusive. Here, we combined a physiologically relevant BE mouse model with multiscale in vivo approaches to explore the functional connection between the gut-brain axis and the reward and homeostatic brain structures. Our results show that BE elicits compensatory adaptations requiring the gut-to-brain axis which, through the vagus nerve, relies on the permissive actions of peripheral endocannabinoids (eCBs) signaling. Selective inhibition of peripheral CB1 receptors resulted in a vagus-dependent increased hypothalamic activity, modified metabolic efficiency, and dampened activity of mesolimbic dopamine circuit, altogether leading to the suppression of palatable eating. We provide compelling evidence for a yet unappreciated physiological integrative mechanism by which variations of peripheral eCBs control the activity of the vagus nerve, thereby in turn gating the additive responses of both homeostatic and hedonic brain circuits which govern homeostatic and reward-driven feeding. In conclusion, we reveal that vagus-mediated eCBs/CB1R functions represent an interesting and innovative target to modulate energy balance and counteract food-reward disorders.

声明:脑医汇旗下神外资讯、神介资讯、脑医咨询、AiBrain所发表内容之知识产权为脑医汇及主办方、原作者等相关权利人所有。未经许可,禁止进行转载、摘编、复制、裁切、录制等。经许可授权使用,亦须注明来源。欢迎转发、分享




