
2020年9月接诊一例来自河南省南阳市的4岁男性患儿(身高106cm,体重16kg)。患儿主因“间断性头痛4天,加重1天”来我院急诊就诊。头颅CT显示“后颅窝巨大占位,梗阻性脑积水,脑疝征象”。查体示:患儿头痛明显,痛苦面容,意识清楚,言语不够流畅,无明显神经系统阳性体征。鉴于患儿后颅窝占位诊断明确,恶性肿瘤可能性大,已合并梗阻性脑积水,出现脑疝前征象,依据天坛小儿神外诊疗常规,当晚急诊行右侧脑室-腹腔分流术。手术过程顺利,术后恢复好,高颅压危象解除。再行头颅MRI显示:后颅窝中线处可见巨大团块状混杂信号影,不均匀强化;瘤体大小约6x5x5cm,髓母细胞瘤,室管膜瘤待除外。
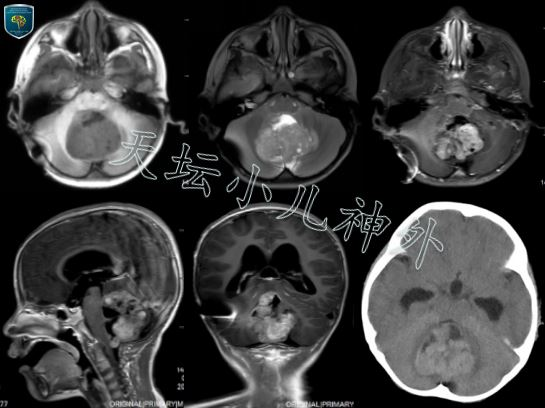
图1.患者术前CT/MRI显示:后颅窝占位,CT呈等密度或稍高密度;MRI呈长T1短T2混杂信号,不均匀强化,瘤体边缘不清,大小约6x5x5cm.
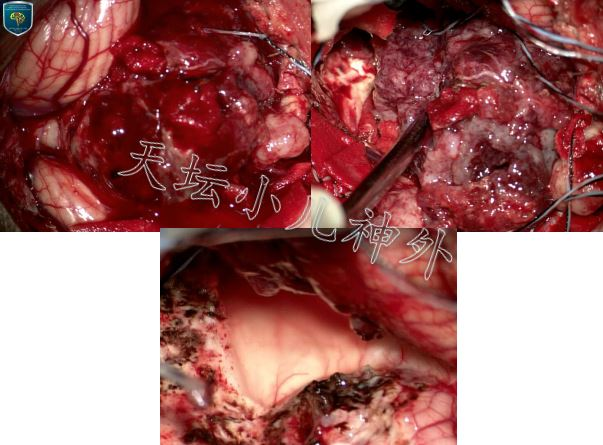
图2.术中所见:肿瘤呈灰红色烂鱼肉样,质地软,血供极其丰富,与小脑蚓部、两侧半球边界欠清晰,与右侧桥臂粘连紧密,脑干背侧中线处有光滑游离面.镜下近全切除.
患儿后颅窝巨大占位诊断明确,完善术前检查,于2020年10月9日在全麻下行后正中入路肿瘤切除术。由于术前分流,术中后颅窝张力不高,打开硬膜,牵开双侧扁桃体,见四室内肿瘤,灰红色、质软烂鱼肉样、血供极其丰富,与小脑蚓部、两侧半球边界不清,与第四脑室底右侧桥臂处粘连紧密,脑干背侧中线处与肿瘤边界清,可见光滑面。电凝切断来源于右侧PICA蚓支的供血动脉后,瘤体出血明显减轻。沿肿瘤周边游离后,完整摘除,瘤体大小6*5*5cm,中脑导水管下口开放良好,后颅窝减压充分,肿瘤近全切除,脑干功能电生理监测显示未见明显手术损伤。术后患儿恢复好,未见新增神经系统阳性体征。术后病理回报提示:AT/RT,非典型畸胎样/横纹肌样瘤(WHO Ⅳ级)。免疫组化结果:Syn(部分+),NeuN(-),Ki-67(约30-50%),GFAP(部分+),NF(-),INI-1(-),BRG-1(+),SMA(+),CD99(+),CK(-),Olig-2(-),Fli-1(-),EMA(+),BCOR(-),H3K27M(-)。术后1周余患儿恢复好,顺利出院,进行后续辅助治疗。
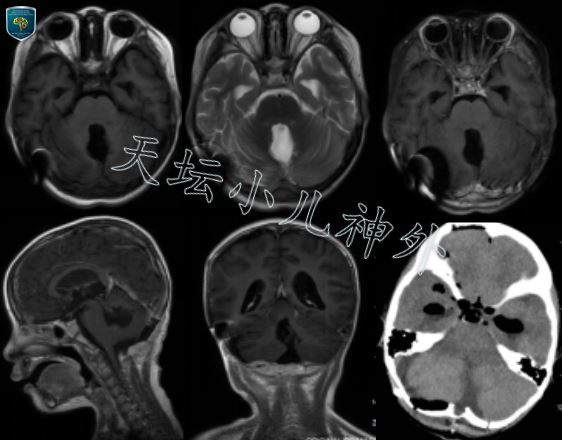
图3 术后头颅CT/MR显示肿瘤切除满意.
治疗体会
WHO(1993)一度将髓母细胞瘤、非典型性畸胎瘤样/横纹肌样瘤(AT/RT)等都归为原始神经外胚层肿瘤(PNET),属高度侵袭、低分化的恶性胚胎性肿瘤1,2。随着在分子水平对中枢神经系统肿瘤的深入研究,最新版WHO(2016)分型,删除了PNET这一分类,将髓母细胞瘤、非典型性畸胎瘤样/横纹肌样瘤(AT/RT)归为胚胎性肿瘤,并将所有胚胎性肿瘤列为IV级肿瘤3,4。AT/RT约占儿童中枢神经系统肿瘤5%,但在小于3岁的婴幼儿中枢神经系统恶性肿瘤中占比高达20%5-7。大约有73%的AT/RT发生于小脑,由于高度恶性,常合并出血坏死,73.3%合并囊性变,40%伴肿瘤钙化8,9。
在分子水平,根据DNA甲基化以及基因表达谱的数据,将AT/RT分为3个亚型:ATRT-SHH (Group 1)、 ATRT-TYR(Group 2A)、ATRT–MYC(Group 2B) 10,11。有研究针对临床数据和分子检测,建立了预后模型:高风险患者:<1岁的非TYR型患者,5年生存率为0;中风险患者:<1岁的TYR型患者或≥1岁的TYR型患者,5年生存率为为32.5%;低风险患者:≥1岁的TYR型患者,5年生存率为71.5%12。
手术尽可能全切肿瘤,是提高AT/RT患儿生存率的前提。术后尽早放疗、化疗可以有效改善预后12-14,提高术后生存率。长春新碱、顺铂、环磷酰胺、依托泊苷是最常用化疗方案;贝伐单抗用于阻断血管内皮生长因子;鞘内注射甲氨蝶呤和拓扑替康也可以纳入治疗方案13,15。本例为3岁以上患儿,实现了肿瘤近全切除,应尽早开始放化疗,可以为患儿争取理想的生存期。
参考文献
1. New Brain Tumor Entities Emerge from Molecular Classification of CNS-PNETs.
2. In Vivo Models for Defining Molecular Subtypes of the Primitive Neuroectodermal Tumor Genome: Current Challenges and Solutions.
3. A Simplified Overview of World Health Organization Classification Update of Central Nervous System Tumors 2016.
4. Kleihues P, Louis DN, Scheithauer BW, et al: The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol 61: 215-225, 2002.
5. Packer RJ, Beigel JA, Blaney S, et al: Atypical teratoid/rhabdoid tumor of the central nervous system: Report on workshop. J Pediatr Hematol Oncol 24:337-342, 2002.
6. Ho DM-T, Hsu C-Y, Wong T-T, et al: Atypical teratoid/rhabdoid tumor of the central nervous system: A comparative study with primitive neuroectodermal tumor/medulloblastoma. Acta Neuropathol 99:482-488, 2000.
7. Hilden JM, Meerbaum S, Burger P, et al: Central nervous system atypical teratoid/rhabdoid tumor: Results of therapy in children enrolled in a registry. J Clin Oncol 22:2877-2884, 2004.
8. Hirth A, Pedersen PH, Wester K, Mörk S, Helgestad J. Cerebral atypical teratoid/rhabdoid tumor of infancy: Long-term survival after multimodal treatment, also including triple intrathecal chemotherapy and gamma knife radiosurgery–Case report. Pediatr Hematol Oncol.2003;20:327–32.
9. Karnes PS, Tran TN, Cui MY, Bogenmann E, Shimada H, Ying KL. Establishment of a rhabdoid tumor cell line with a specific chromosomal abnormality, 46, XY, t(11;22)(p155;q1123) Cancer Genet Cytogenet.1991;56:31–8.
10. Johann PD, Erkek S, Zapatka M, et al: Atypical Teratoid/Rhabdoid Tumors Are Comprised of Three Epigenetic Subgroups with Distinct Enhancer Landscapes. Cancer Cell. 2016;29(3):379- 393.
11. Torchia J, Golbourn B, Feng S, et al: Integrated (epi)-Genomic Analyses Identify SubgroupSpecific Therapeutic Targets in CNS Rhabdoid Tumors. Cancer Cell. 2016;30(6):891-908.
12. Frühwald MC. Age and DNA methylation subgroup as potential independent risk factors for treatment stratification in children with atypical teratoid/rhabdoid tumors. Neuro Oncol. 2020 Jul 7;22(7):1006-1017.
13. Tekautz TM, Fuller CE, Blaney S, et al. Atypical teratoid/rhabdoid tumors (ATRT): improved survival in children 3 years of age and older with radiation therapy and high-dose alkylator-based chemotherapy. J Clin Oncol. 2005;23:1491-1499.
14. Schrey D, Carceller Lechon F, Malietzis G, et al. Multimodal therapy in children and adolescents with newly diagnosed atypical teratoid rhabdoid tumor: individual pooled data analysis and review of the literature. J Neurooncol. 2016;126:81-90.
15. Rios CI, De Jesus O. Primitive Neuroectodermal Tumor. 2020 Nov 14. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan.






