近日,经过欧盟CE认证机构的严格审查,通桥医疗自主研发的Thrombite™(蛟龙)取栓支架获得欧盟CE注册认证,成为同类国产产品迈向欧洲高端医疗器械市场的先行者之一,标志着通桥医疗“中国匠造”正式开启海外征程。
Recently, after the strict audit by an European Union CE certification agency, Thrombit™ (蛟龙) clot retriever device, independently developed by Tonbridge Medical, successfully obtained the CE medical certification. The device becomes one of the domestic device pioneers that are available in the European high-end medical device market. This is a milestone for Tonbridge Medical to officially start its overseas journey with the Chinese Craftsmanship spirit.
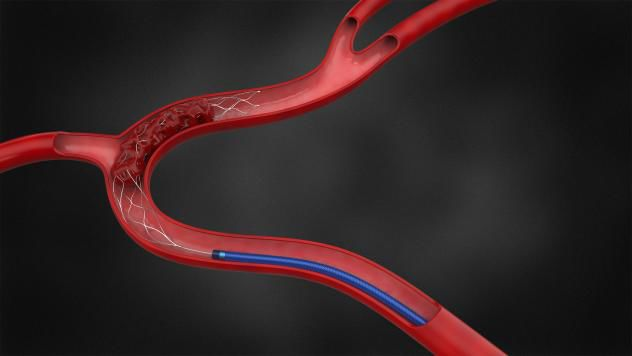
Thrombite™(蛟龙)取栓支架通过机械性消除颅内血栓以达到恢复血流的治疗目的。其独特的侧边螺旋上升的开放结构,将血栓旋转缠住,对内嵌的血栓加持力更强,血栓不易脱落。该技术特点即将获得中国和欧盟范围内具有自主知识产权的专利,并在研发和临床试验过程中,备受欧洲、亚太和南美等众多国家临床专家的高度关注。产品取得CE认证后,市场销售和应用前景看好。
The Thrombite™ clot retriever device can restore blood flow by mechanically removing the intracranial thrombus. Its unique spiral-shape side opening structure can more efficiently wrap the thrombus. This design enhances the catching capability of the stent resulting the more successful removal rate of the thrombus. This unique technology is applying patents globally. In the meanwhile, the technology and clinical trials is gaining more and more attention from physicians in Europe, Asia, South America, etc. After the product obtained CE certification, we believe the sale and clinical application are very promising.
根据《中国脑卒中防治报告2018》,脑卒中是我国国民的第一位死亡原因,我国每12秒就有一人发生脑卒中,每21秒就有一人死于脑卒中。《中国心血管病报告2016》显示,目前我国有1300万左右的卒中患者,每年新发病人达到约200万,患病人数年化增长率达到15%。Thrombite™(蛟龙)取栓支架作为国家药品监督管理局(NMPA)III类“创新医疗器械”绿色通道产品,已经完成中国多中心随机对照注册临床试验,在临床试验中和全球销量领先的对标产品比较展现出了卓越的产品特性,即将在中国上市。
According to the Report on Stroke Prevention and Treatment in China (2018), stroke is the first cause of death in China, where stroke occurs every 12 seconds, and a death occurs every 21 seconds. Report on Cardiovascular Diseases in China (2016) shows that there are currently about 13 million stroke patients in China with an annual growth of 2 million new patients. The growth rate is more than15%. Thrombite™ clot retriever device, as a class III National Innovative Medical Device with a “green channel” granted by Chinese National Medical Products Administration (NMPA), has completed a multi-center randomized, controlled and registered clinical trial in China, in which the device shows excellent clinical performance, compared with compatible products with leading global sales volume. The product will soon be available in Chinese market.
本次CE认证的取得和欧洲市场的快速推广,将积累更多的海外使用经验,更丰富的产品安全性和有效性数据,为国内专家的使用提供重要参考。今年,通桥医疗将携Thrombite™(蛟龙)取栓支架亮相在奥地利举办的ESO-WSO 2020大会(欧洲卒中组织和世界卒中组织联合大会),用自己的小小跬步向全世界展现中国高端医疗装备的千里长卷!
The acquisition of the CE certification and the rapid promotion in the European market will accumulate more overseas user experience as well as more product safety and effectiveness data, providing very valuable references for the coming use in China. This year, TonBridge Medical will also bring the Thrombite™ clot retriever device to the Joint Meeting of European Stroke Organization and World Stroke Organization in Austria, ESO-WSO 2020. We are ready to show our device to the world as one of the Chinese cutting-edge medical equipment!
宝剑锋自磨砺出,2020年,刚刚度过四周岁生日的通桥医疗迎来销售元年。继Thrombite™(蛟龙)取栓支架之后,通桥医疗的颅内支持导管也将于近期推出,包括脑卒中缺血线和出血线的各系列产品的布局和临床进展,将形成一个完整的颅内神经血管介入高端医疗器械产业平台。此前,归创医疗生产的外周球囊扩张导管和外周支架系统等产品已远销欧洲,此次Thrombite™(蛟龙)取栓支架的获批将补充归创通桥血管介入和植入体系的海外产品线。我们期待更多用于治疗出血性和缺血性脑卒中的通桥产品出现在海内外市场,给海外患者提供有自主知识产权和高质量全线神经血管介入解决方案。





